20 April 2013 |
Oberflächen POLYSURFACES 06/2012 |
Korrosionsschutz
Corrosion protection with «perfect» atomic layers
Prof. Erich Bergmann and David Rosello
Since metallic alloys are used to produce parts, people have to care about corrosion problems and several techniques have been developed to fight against those. Of course there are metals that have natural well-known corrosion resistance properties, but it would be in some cases too expensive to have the whole part made of those materials. The best option is to build the part with an easy working and cheap material and apply a thin protective layer at its surface to prevent corrosion.
CORRAL was a European research project started in 2008 and ended in August 2011. The project involved a consortium of European Universities and has been coordinated by the HES-SO under management of Prof. Erich Bergmann (HES-GE) and Dr. Daniel Morel (HE-ARC). The aim of this project is to develop high density defect-free ultra-thin sealing coatings with excellent barrier properties and improved corrosion resistance. To reach our objective, different coating processes have been tested. The study has quickly been focused on the development nanoscale multilayers. These impermeable sealing layers are able to block the ion exchange between the substrate material and an aggressive environment, thus offering an efficient protection against corrosion over a long term. Some surface pre-treatment at the macro-scale have also been tested.
Deposition techniques
Different processes to apply CORRAL coatings have been studied during the project. Some techniques have been abandoned due to bad results and properties of the coatings. This chapted presents the techniques which have given the best results.
HIPMS and dual magnetron sputtering
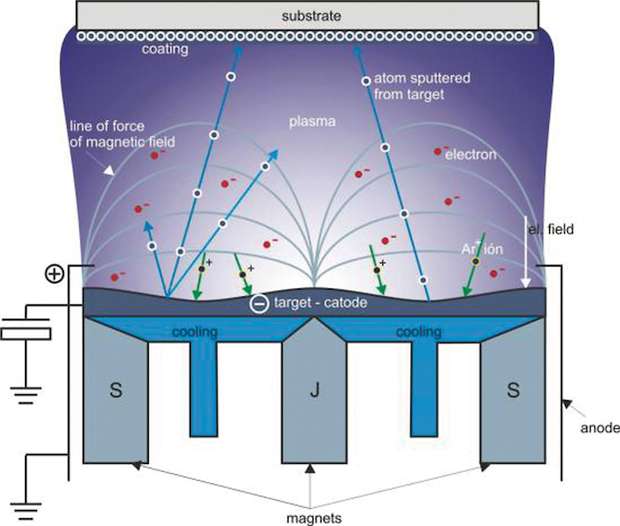
Magnetron sputtering is a vacuum deposition technique where a substrate is placed in a chamber at a pressure around 10-5 mbar in front of a target made of the material we want to deposit. A small flow (between 10 and 100 sccm, depending on the chamber geometry) of neutral gas is introduced in the chamber (typically Argon) and a strong DC, Radio Frequency or high impulse current is applied to the cathode (the target). The neutral gas atoms are energised by the electric field and plasma ignites. The magnetic field generated by the permanent magnets keeps the atomic species of the plasma along its field lines of force and heavy ions of the neutral gas are projected against the target. Atoms sputtered from the target escape from the plasma and deposit at the surface of the substrate (fig. 1).
HIPMS is a classical magnetron sputtering technique but we use high impulse generators to apply the potentials to our system. The plasma species have then bigger energy but in small periods of time. Plasma is very energetic without the inconvenient of constant current plasmas which tends to heat the target on critical ways.
The Sheffield Hallam University (SHU) has a dual magnetron and HIPMS chamber and some CORRAL coatings were developed with this technique. The advantage of HIPMS is that the sputtered atoms have bigger kinetic energy. We can also use HIPMS for adhesion-enhancing pre treatment of the substrates prior to coating deposition (substrate etching) and the deposition of thin films has high microstructure density.
Filtered Cathodic Arc Deposition (FCAD)
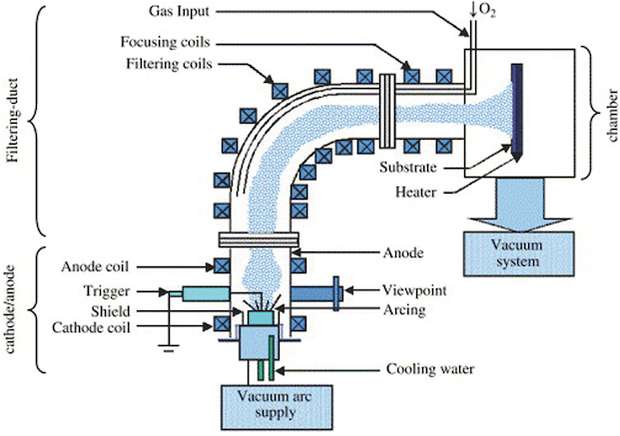
Cathodic Arc Deposition is a plasma deposition technique where we use the energy of an electric arc to evaporate some metals from the target to the substrate. This kind of evaporation is very energetic and this process is considered as a «hot» plasma coating process (by «hot» we mean that the species of the plasma have all high energies, by opposition to cold plasmas on which electrons only have high energies). The electric arc hits the target and sputter atoms.
The inconvenient of this technique is that the sputtered particles are very irregular due to the high energy of the impact. They can be ions, atomic or nanoparticles that sometimes are unwanted. There is no simple way to eliminate macro-particles from the depositing plasma flow in the direct arc source configuration, since the operating surface of cathode target is in line of sight with the substrate surfaces to be coated. These macro-particles deleteriously influence critical properties of the coatings. An effective way to eliminate unwanted particles is to add a «filter» to the device, which will select and eliminate unwanted particles from our chamber (fig. 2).
The 90° tube connects the source to the substrate chamber. The sputtered particles are going through this tube and tend to follow the electromagnetic field lines produced by the coil. Light and charged particles can easily cross the tube and heavy neutral particles impact the sides of the tube. With this technology, only well selected species arrive to the substrate and it grants very impurities-free layers with high deposition-rate.
Atomic Layer Deposition (ALD)
ALD is a vacuum deposition technique based on alternate surface reactions accomplished by dosing gaseous precursors successively and purging the chamber between each cycle. When the precursors and the parameters are tuned in an optimal way, the surface reactions cover the entire part surface and saturate it guaranteeing a complete sealing of the part. This technique ensures excellent coating conformity, and atomic monolayer film growth control. In ALD, film growth takes place in a cyclic manner. In the simplest case, it takes place in four steps:
- Exposure of the first precursor
- Purge of the reaction chamber
- Exposure of the second precursor
- Purge or evacuation of the precursor
The working mechanism is illustrated in figure 3, taking example of an aluminium oxide deposition. The cycle is repeated as many times as necessary, and at every cycle we add an atomic layer of stable coating. The film thickness can be accurately controlled simply by the number of deposition cycles. In a majority of processes, reactions are activated thermally in isothermal conditions (typically around 250 degrees). It must be recognized that during the very first cycles when the substrate is converted from the substrate to the film material the growth rate can vary due to the surface density, the chemisorptions and the reactive sites. The self-limiting growth mechanism also ensures that the precursor fluxes do not need to be uniform over the substrate surface.
Typically, ALD processes have a film growth rate of 100 to 300 nm per hour. The main weakness of this technique is of course its slowness. For CORRAL coatings, it was not a problem thanks to our layers thickness requirements, typically between 10 and 60 nm.
Corrosion tests
Inox FeCl3(ASTM G48)
For corrosion tests on stainless steel samples, NSS spray test is not sufficient to oxidize the surface and test corrosion resistance of the sample. The ASTM G48 test evaluates an alloys resistance to pitting and crevice corrosion using severe test conditions. The principal alloying elements for corrosion resistance of the high-alloyed stainless steels are increased chromium content, which provides progressive resistance to general attack; andincreased molybdenum (together with small additions of nitrogen), which enhances resistance to localized attack. Some of these alloys, despite high nickel, chromium, and molybdenum contents, may besusceptible to localized corrosion in certain environments. We had the idea to protect parts made with those alloys to corrosion with the sealing properties of CORRAL coatings, and this test was chosen for all stainless steels used during the test phase (SS316L and SS304). The test parameters are:
- FeCl3solution: 100 g FeCl3*6H2O in 900 ml distilled water
- Temperature: 85 °C
- pH value (adjustment with hydrochloric acid): ≤1
- Test duration: 72 h
The temperature was increased with respect to the ASTM standard because increasing the temperature of the ferric chloride solution decreases its pH. At a critical temperature of 45 °C ferric hydroxide begins to precipitate and the solution rapidly becomes more acidic. This change in solution pH will affect its corrosivity, particularly to lesser alloyed stainless steels.
There are many factors, which are known to cause localized corrosion of stainless steels; among these are low pH, high chloride concentrations, and solutions with noble redox potentials, oxygen differential concentrations, and elevated temperature.
Neutral Salt Spray (NSS)
NSS tests are described in the European Standard EU ISO 9227 or German Standard DIN 50021. It is a method used to verify if the comparative quality of corrosion resistance to salty mediums between coated and uncoated samples is maintained. The method consists in spraying a NaCl solution on the sample and observes the evolution of the corrosion. The concentration of the solution is 50 ±5 g/l of NaCl in 20 µS/cm à (25 ±2 °C deionized water. The NSS tests in for the parts of CORRAL project were made by our partner FEM in Germany. The parameters in the chamber are
- Electrolyte: 50 ±5 g/l NaCl à 0,86 M NaCl
- pH value: 6,5 to 7,2
- Temperature: 35 ±2 °C
The neutral salt spray test was performed according to the standard DIN 50021, ISO 9227 but with the deviation, that the samples were rinsed in deionized water and dried with a hair dryer (warm not hot air) before the photographs have been taken. If no, or only negligible corrosion products were visible on the samples, the samples have been transferred back to the salt spray test chamber and the test was continued until significant red rust was visible on the 100Cr6 steel samples. Then these samples have been removed from the test chamber.
Coatings morphology
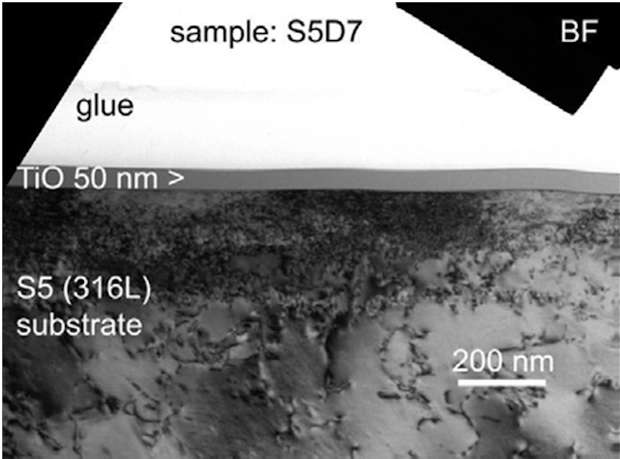
This chapter will show some examples of coatings developed during CORRAL and present the major achievements in terms of coatings structures with respect to the coating technologies. On figure 4 we can see a dense and regular coating. FCAD coatings are typically very pure due to species filtration system between the source and the chamber.
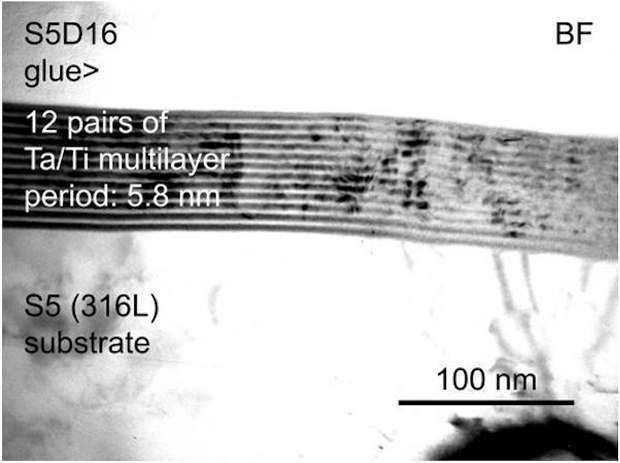
Another tested coating morphology were nanolaminates. A fine coating combining properties of two types of coatings by stacking layers of different compositions. Technologically speaking, having a nanolaminate with those dimensions and conformity is a real challenge. Figure 5 shows a 70 nm nanolaminate layer deposited by FCAD on 316L substrate. The layers are made of metallic Ti and Ta. The thickness of the each layer is about 3 nm and we can observe that the growing of the coating is not very regular as it is influenced by the substrate. Thin deep cracks will be very difficult to coat with this type of coatings. The irregularities in the coating on the right part of the image could also be due to the cutting of the sample prior to analysis.
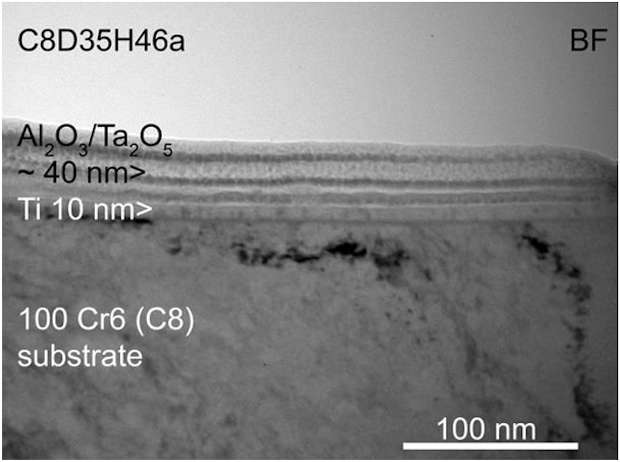
Another type of tested coatings was a combination of the two previous coatings, wich means a first metallic layer, for adhesion and little diffusion barrier, and a second composite layer or nanolaminate, for enhaced diffusion barrier and anticorrosion properties (fig. 6). This family of CORRAL coatings has been called «Duplex» coatings because they are the result of a combination of two coating technologies and different layers. This example is a 100Cr6 substrate coated with a 10 nm metallic layer and around 40 nm of an Al-Ta:O layer deposited by ALD technology. To enhance adhesion on 100Cr6 Substrates, and especially on carbide grains, we can also change the metallic adhesion layer by a reactive FCAD layer.
In figure 7 we can see a «Duplex» coating on an 100Cr6 substrate with a FCAD Cr:O/Ta:O composite adhesion layer. The adhesion is optimal and regular even on carbide grains surface. The second layer is a 20 nm pure alumina layer deposited by ALD.
Major Achievements
This chapter shows the major achievements in terms of sealing properties and in corrosion protection of electroplated layers.
Sealing properties
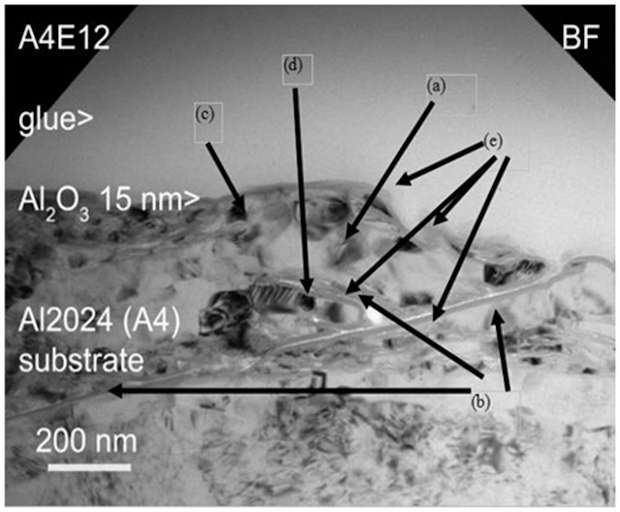
As the best way to protect a part of corrosion is to «package» it with an hermetic coating, sealing properties were really wanted and the objective was to prove that sealing is efficient. The more the surface is rough, the more it becomes hard to cover the part totally. On very small cracks, high eneretic coating techniques will overcome the holes and the coating will become fragile at those points. The coating technique offering the best sealing properties was without a doubt ALD in terms of conformity and distribution. During a routine analysis of an ALD coating, this image was taken (it became the logo of the project), showing the impressive sealing properties of ALD coatings.
Figure 8 shows a 15 nm of Al2O3deposited by PEALD on a ground surface of a flat manufactured from Al2024 illustrating best the problems and achievements: The poor quality of the grinding has produced a large burr (a) that was folded onto the surface creating a deep narrow gap (b); around 40 nm. The burr has been work hardened (c) by the plastic deformation. An abrasive grinding particle (d) has also been incorporated by the bur. The 15 nm amorphous Al2O3coating (e) deposited by PEALD covers completely all features even the walls of the narrow gaps providing perfect sealing. The figure illustrates the problems to be tackled and the achievements. The project has made progress on all three challenges:
- Preparing, observing and controlling engineering surfaces at nanoscale
- Developing technologies for coatings that are perfect at nanoscale and can handle and seal the residual defects
- Studying corrosion initiation and development at nanoscale
Anticorrosion enhancements of electroplated layers
NSS tests were done on 100Cr6 samples that were galvanic coated by Schaeffler Technologies GmbH & Co KG, a partner company specialized in galvanic coatings. Tests were made on three types of galvanic coatings, and research was pushed in the corrosion resistance enhancement properties way.
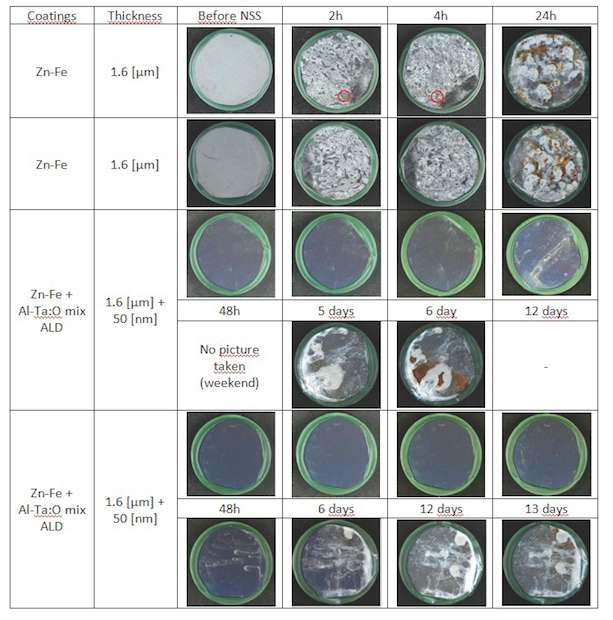
Zn-Fe coatings: The NSS tests made on Zn-Fe 100Cr6 galvanic coated samples gave the results in figure 9. On this Zn-Fe coated samples, we can see that added ALD coating improves corrosion resistance of the sample from 1 day to 5 to 13 days. That makes a factor 5 to 13 in corrosion resistance improvement.
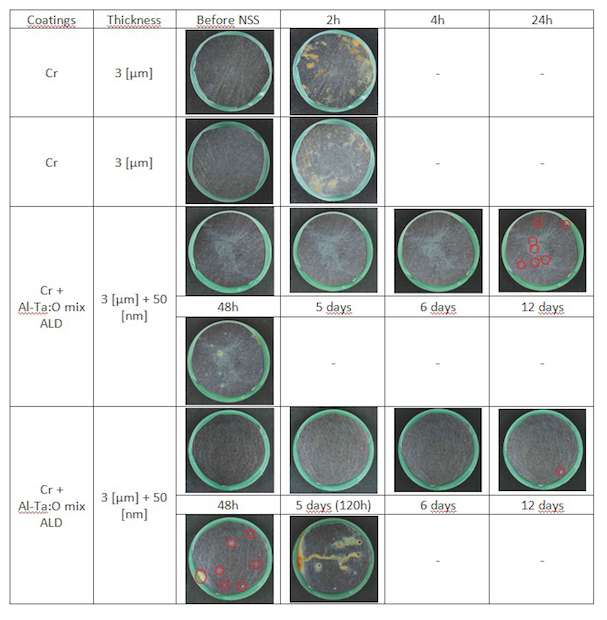
Cr coatings: On the Cr coated samples in figure 10 we can see that added ALD coating improves corrosion resistance of the sample from 2 h to 1 to 2 days. That makes a factor 12 to 24 in corrosion resistance improvement. Corrosion spots appear after 24 h.
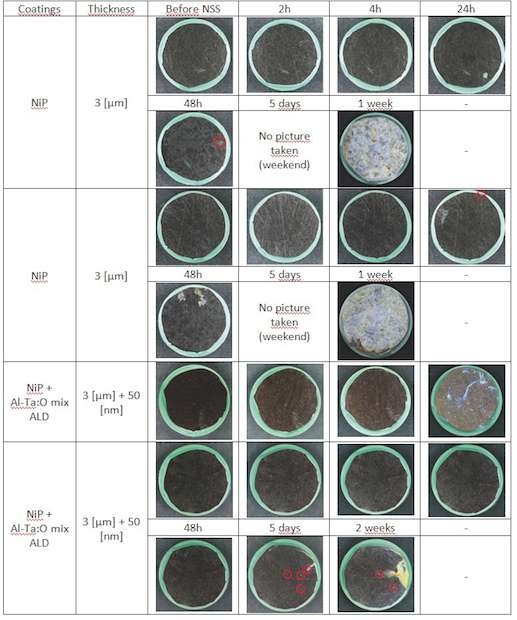
NiP coatings: NiP coated samples are very resistant to corrosion (fig. 11). We can see that in the first case, corrosion resistance has decreased. This is theorically impossible, unless the NiP coating has been damaged during ALD coating process (too high temperature or mistreatment of the sample). In that case we have no explanation. In the second case, added ALD coating improves corrosion resistance in from 48 h to 5 days. That makes a factor 2.5 in corrosion resistance improvement. However, after two weeks of NSS testing this sample has only a few corrosion sites (1 or 2 of them are bleeding) compared to the severe corrosive attack of the NiP coated samples after one week.
For all samples a significant improvement of the corrosion behavior is obtained by sealing with an ALD Al-Ta-O mixture coating. The biggest improvements are found for ALD sealing of ZnFe and Cr coatings.
|
Zusammenfassung
2008 hatte die HES-SO im Rahmen des 7. Rahmenprogramms das Europäische Projekt CORRAL (CORRosion protection with perfect Atomic Layers) gestartet. Das Ziel dieses Forschungsprojekts war es, ultradünne Schichten im Nanometerbereich zu entwickeln, die den so beschichteten Komponenten einen hohen Korrosionsschutz bieten sollen. Bei den verwendeten Beschichtungsverfahren handelte es sich um FCAD (Filtered Cathodic Arc Deposition) und ALD (Atomic Layer Deposition). Sie erlauben die Abscheidung defektfreier Schichten. Die entwickelten Schichten sind aus Tantal- und Aluminiumoxid aufgebaut und haben eine Schichtstärke, die je nach Anwendung im Bereich von 15 bis 100 nm liegt.
Korrosionsversuche wurden auf 100Cr6, X155CrVMo12, Al2024 und 316L mit verschiedenen mechanischen und plasmachemischen Vorbehandlungen und Schichtarchitekturen durchgeführt. Als Korrosionstests wurden neutraler Salzsprühnebel (DIN 50021) für den Bau- und den Werkzeugstahl sowie die Aluminiumlegierung und der 72-h-Eisenchloridtest (ASTM G48) angewendet. Die Resultate sind eindrücklich. Beim Eisenchloridtest für rostfreien Stahl übertreffen die ultradünnen CORRAL-Beschichtungen die zurzeit verwendeten Dickschicht-Korrosionsschutzschichten. Gleiches gilt für 100Cr6. Ein überraschendes Resultat ist, dass CORRAL-Schichten andere Beschichtungen wie PVD und Galvanik vollkommen korrosionsfest machen. Damit wird erstmals eine verschleiss- und korrosionsfeste Beschichtung möglich.
|
|
Résumé
Durant l’année 2008, la HES-SO en partenariat avec le 7th Framework Program ont lancé le projet Européen CORRAL (CORRosion protection with perfect Atomic Layers). Il s’agit d’un projet de recherche visant à mettre au point des revêtements à l’échelle nanométrique offrant une haute résistance à la corrosion aux substrats sur lesquels ils sont déposés. Les technologies utilisées pour effectuer nos revêtements sont appelées FCAD (Filtered Cathode Arc Deposition) et ALD (Atomic Layer Deposition). Ces procédés de déposition, nous permettent d’obtenir des couches sans défaut et quasiment monocristallines. Nos revêtements sont essentiellement composés d’oxydes de tantale et d’aluminium et ont une épaisseur pouvant varier entre 15 et 100 nm.
Des tests de corrosion poussés ont été effectués sur des substrats de 100Cr6, Al2024 et Acier Inox 316L ayant subi différents prétraitements (physiques et/ou plasma), puis revêtus par nos traitements CORRAL. La corrosion s’effectue par une immersion de 72 h dans du chlorure de fer (III) selon les normes ASTM G48 (acier inox) et des tests climatiques pour le 100Cr6 et Al2024. Les résultats, obtenus jusqu’à présent, sont très prometteurs, dans le cas des essais sur Al2024, nous arrivons à deux piqûres après une semaine d’attaque dans le meilleur des cas. Pour le 100Cr6, une corrosion beaucoup plus sévère peut être remarquée sur les échantillons non-traités. Enfin, pour l’acier Inox 316L, aucune corrosion n’est observée après 72 h d’immersion dans du FeCl3 pour les pièces traitées FCAD + ALD.
|
Prof. Dr. Erich Bergmann
David Rosello
Haute Ecole Spécialisée de Suisse Occidentale (HES-SO)
Laboratoire de Nanotechnologie hepia
Rue de la Prairie 4
1202 Genève
Tél. 022 546 68 00
david.rosello@hesge.ch
www.hesge.ch


 Abonnements
Abonnements